If you supply medical devices to the USA, Europe and China you must comply with Unique Device Identification (UDI) regulations.

Being able to identify, track and trace medical devices at a global level is important for patient safety. To ensure compliance, the US FDA (Food and Drug Administration), European Union's Medical Device Regulation (MDR), China's National Medical Products Administration and many other countries has established a Unique Device Identification (UDI) system for medical devices.
Suppliers and manufacturers of medical devices must comply with new regulations. These state that all medical devices entering the healthcare supply chain must be identified with a UDI.
GS1 standards conform to all UDI requirements, as such we are recognised as the first accredited UDI Issuing Agency in many countries.
UDI compliance dates
Below are the compliance dates for UDI implementation for each country and region:
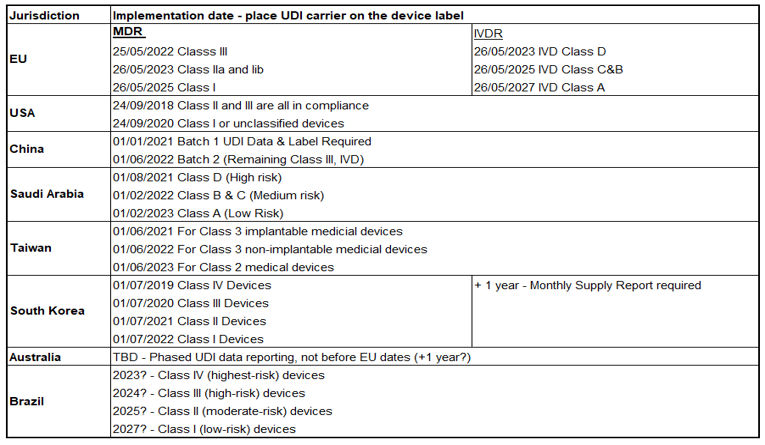
What does the UDI regulation mean for you?
You must make sure your products are in compliance before they are shipped / traded.
Using GS1 standards to enable compliance
GS1 Global is an accredited issuing agency for UDI by US FDA, EU, China, South Korea and Brazil, our standards for product identification and barcoding conform to UDI requirements, including:
- Product identification at each level of the packaging hierarchy
- The barcode symbols for medical devices
- Device and Production information included together
- Product technical data must be stored in the US UDI Database (GUDID) and the EU UDI Database (EUDAMED)
How GS1 Malaysia can help
As the experts on GS1 standards, we are ideally placed to help you comply with the FDA mandate, and ensure you will comply with EU requirements that will follow. Our range of training and implementation services is helping medical device manufacturers of all sizes to comply, but also operate more efficiently and save money.


Contact GS1 Malaysia at gs1mymembership@googlegroups.com to learn more about UDI and arrange to attend our industry focus forums on UDI (kindly note that the industry focus forums are chargeable sessions).

